Regulatory Affairs

We possess capabilities for preparing, Technical Dossiers and Drug Master Files (DMFs) for a range of our products. Our facilities are constantly upgraded to comply with European and US Regulatory inspections, making us strategically poised to enter Regulated Markets and ROW Markets.
Registered Products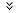
No. |
Country |
Products Registerd |
1 |
Jamaica |
96 |
2 |
Panama |
6 |
3 |
Trinidad & Tobago |
13 |
4 |
Honduras |
30 |
5 |
Cuba |
30 |
6 |
DR |
29 |
7 |
Philippines |
11 |
8 |
Bolivia |
3 |
9 |
Elsavador |
12 |
10 |
Guyana |
86 |
11 |
Nigeria |
2 |
12 |
Costa Rica |
3 |
13 |
Maldives |
6 |
14 |
Nicaragua |
4 |
15 |
Sri Lanka |
18 |
16 |
Turkmenistan |
4 |
17 |
Kyrgyzstan |
5 |
18 |
Guatemala |
4 |
19 |
Myanmar |
1 |
20 |
Paragway |
6 |
21 |
Botswana |
1 |
22 |
Bhutan |
3 |
23 |
Cambodia |
4 |
24 |
Mongolia |
7 |
25 |
Namibia |
1 |
26 |
Mauritius |
9 |
27 |
Tajikistan |
3 |
TOTAL |
397 |
Submitted Products 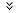
No. |
Country |
Products Sumitted |
1 |
Jamaica |
12 |
2 |
Panama |
8 |
3 |
Trinidad & Tobago |
15 |
4 |
Honduras |
37 |
5 |
Cuba |
6 |
6 |
DR |
31 |
7 |
Guyana |
30 |
8 |
Costa Rica |
4 |
9 |
Nicaragua |
8 |
10 |
Sri Lanka |
10 |
11 |
Turkmenistan |
1 |
12 |
Guatemala |
16 |
13 |
Myanmar |
24 |
14 |
Botswana |
4 |
15 |
Bhutan |
9 |
16 |
Cambodia |
4 |
17 |
Mongolia |
6 |
18 |
Peru |
1 |
19 |
Burundi |
3 |
20 |
Namibia |
9 |
21 |
Vietnam |
1 |
22 |
Ecuador |
3 |
23 |
Peru |
1 |
24 |
Burundi |
3 |
25 |
Zimbabwe |
3 |
26 |
Laos |
5 |
27 |
Malaysia |
1 |
TOTAL |
255 |
Pipeline Products 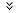
Sr No |
Country |
Products in Pipeline |
1 |
Jamaica |
25 |
2 |
Panama |
10 |
3 |
Trinidad & Tobago |
1 |
4 |
Honduras |
26 |
5 |
Cuba |
58 |
6 |
DR |
10 |
7 |
Philippines |
2 |
8 |
Bolivia |
5 |
9 |
Guyana |
5 |
10 |
Nigeria |
4 |
11 |
Costa Rica |
36 |
12 |
Nicaragua |
12 |
13 |
Sri Lanka |
15 |
14 |
Guatemala |
27 |
15 |
Myanmar |
25 |
16 |
Bhutan |
15 |
17 |
Azerbaijan |
5 |
18 |
Cambodia |
18 |
19 |
South Africa |
4 |
20 |
Mongolia |
2 |
21 |
Peru |
3 |
22 |
Burundi |
2 |
23 |
Namibia |
5 |
24 |
Armenia |
13 |
25 |
Togo |
5 |
26 |
Kenya |
3 |
27 |
Tajikistan |
1 |
28 |
Peru |
3 |
29 |
Burundi |
2 |
30 |
Zimbabwe |
5 |
31 |
Laos |
5 |
32 |
Syria |
6 |
33 |
Madagascar |
13 |
TOTAL |
371 |
CTD Dossiers
Ear And Eye Drops 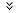
No. |
Product (Ear And Eye Drops) |
| 1 | METRONIDAZOLE INJECTION USP - 0.5% w/v |
| 2 | Paracetamol INV INF 10mg /ml |
| 3 | CYCLOPHOSPHAMIDE FOR INJECTION USP 1g/Vial |
| 4 | Dexamethasone Sodium phaosphate Inj BP 4mg /ml |
| 5 | Nitroglycerin Inj USP 5mg /ml |
| 6 | Sodium Chloride INV INF BP 0.9% |
| 7 | Oxytocin Inj BP 5IU/ml |
| 8 | Progesteron Inj USP 25mg /ml |
| 9 | Propofol Injrction BP 10mg /ml |
| 10 | Ringer lacatate INV INF ( Compound Sodium Lactate Intravenous Infusion BP) |
| 11 | Dextrose INV INF 5% USP |
| 12 | Dextrose INV INF 25% USP |
| 13 | Aciclovir Sodium For Inj BP INV INF 250mg |
| 14 | AMPHOTERICIN- B FOR INJECTION USP 50 mg |
| 15 | Ceftadizime For Inj USP 1g |
| 16 | Cyproheptadine tab BP 4mg |
| 17 | Vitamin B Complex Injection IH |
| 18 | Amikacin Inj USP 500mg |
| 19 | Atracurium Besilate Inj USP 10mg |
| 20 | Azithromycin For Inj 500mg |
| 21 | CLINDAMYCIN PHOSPHATE INJECTION 150 mg BP |
| 22 | DEXTROSE INJECTION 50 % w/v USP |
| 23 | Ergometrine maleate Inj BP 0.5mg /ml |
| 24 | frusamide Inj BP 10mg/ml |
| 25 | Heparin Injecion BP 1000IU |
| 26 | Heparin Injecion BP 5000IU |
| 27 | Pantoprazol For Inj 40mg IH |
| 28 | Raboprazole For Inj 20mg IH |
| 29 | Vancomycin Hycdrochloride For Inj 1g |
| 30 | Vancomycin Hycdrochloride For Inj 500mg |
| 31 | VECURONIUM BROMIDE FOR INJECTION 4 mg |
| 32 | Chloramphenicol Ear Drop BP 5% |
| 33 | Chloramphenicol Opthalmic Solution USP 0.5% |
| 34 | Ciprofloxacin Opthalmic Solution USP 0.3% |
| 35 | CITICOLINE INJECTION 250 mg/ml IH |
| 36 | AMPHOTERICIN- B FOR INJECTION USP 50 mg |
| 37 | DOCETAXEL INJECTION 20 MG/0.5 ML |
| 38 | DOCETAXEL INJECTION 80 MG/2 ML |
| 39 | MENOTROPHIN FOR INJECTION BP 75 IU) |
| 40 | Esomeprazole For Inj 40mg |
| 41 | Teicoplanin for injection 400 mg |
| 42 | Bleomycin for Injection USP 15 IU |
| 43 | Docetaxel Injection Concentrate 80 mg/ 2.0 ml |
| 44 | Iopamidol Injection USP 300 mg |
| 45 | OCTREOTIDE INJECTION 50 mcg |
| 46 | Pemetrexed For Injection 500 mg |
| 47 | PROGESTERONE INJECTION USP 100 mg |
| 48 | PROGESTERONE INJECTION USP 50 mg |
| 49 | PROGESTERONE INJECTION USP25 mg |
| 50 | Tigecycline for Injection 50mg per ml |
| 51 | Tigecycline for Injection 50mg per ml |
| 52 | TOBRAMYCIN INJECTION USP 40 MG |
| 53 | Dexamethasone Eye Ear Drops /Dexamethasone Sodium Phaosphate Inj USP 0.1% |
| 54 | Docetaxel Injection Concentrate 20mg /0.5ml |
| 55 | CARBOPLATIN INJECTION BP 150mg per 15ml |
| 56 | CARBOPLATIN INJECTION BP 450mg per 45ml |
| 57 | Dimenhydrinate Injection USP 50 mg |
| 58 | Etoposide Injection USP 100 mg/ 5.0 mL |
| 59 | Omeprazole for Injection 40 mg |
| 60 | Fluphenazine Decanate Inj BP 25mg per ml |
| 61 | HALOPERIDOL INJECTION BP 5 mg |
| 62 | HYDRALAZINE HYDROCHLORIDE INJECTION USP 20 mg/ml |
| 63 | Hydrocortisone Sodium Succinate For inj BP 1oomg |
| 64 | Iron dextran Inj USP 50mg per ml |
| 65 | IRON SUCROSE INJECTION USP 20mg per ml |
| 66 | Leucovorin Calcium Injection USP 50 mg/ 5.0mL |
| 67 | Methotrexate Injection BP 50 mg/ 2.0mL |
| 68 | Methyl Prednisolone Sodium Succinate Injection USP 500mg |
| 69 | Methyl Prednisolone Sodium Succinate Injection USP 1g |
| 70 | Paclitaxel Injection USP 30 mg. / 5 ml |
| 71 | Paclitaxel Injection USP100 mg./ 16.7 ml |
| 72 | Paclitaxel Injection USP 260 mg./ 43.4 ml |
| 73 | TOBRAMYCIN opthalmic solution USP 0.3% |
| 74 | DACARBAZINE FOR INJECTION USP 200mg |
| 75 | NITROGLYCERINE INJECTION USP 5mg per ml |
| 76 | SUXAMETHONIUM CHLORIDE INJECTION BP |
| 77 | Gentamycin Eye ear drops 0.3% |
| 78 | Gemcitabine for Injection USP 1 g |
| 79 | Gemcitabine for Injection USP 2oomg |
| 80 | Teicoplanin for Injection 400 mg |
| 81 | Zoledronic Acid for Injection 4.0 mg |
| 82 | Salbutamol Pressurised Inhalation BP 100mcg |
| 83 | Beclometasone Pressurised Inhalation BP 50mcg |
| 84 | DEXAMETHASONE SODIUM PHOSPHATE INJECTION USP 4 mg/mL |
| 85 | Lidocaine Hydrochloride Injection USP 20mg |
| 86 | Caspofungin Acetate for Injection 50 mg |
| 87 | Caspofungin Acetate for Injection 70 mg |
| 89 | Ranitidine Injection USP 25mg |
| 90 | THIOPENTONE SODIUM FOR INJECTION BP 500 mg |
| 91 | MENOTROPHIN FOR INJECTION BP 150 IU |
| 92 | Neostigmine Metilsulfate Inj BP 0.5mg per ml |
| 93 | Promethazine HCL Inj BP 25mg per ml |
| 94 | Rabeprazole for Injection 20 mg |
| 95 | Bupivacaine Injection BP 5 mg |
| 96 | Oxyplatin Injection 100mg |
| 97 | Oxaliplatin Injection 50mg/25ml |
| 98 | Hydrocortisone Sodium Succinate for Injection BP 500 mg |
| 99 | Protamin Sulphate Inj BP 10mg |
| 100 | Pralidoxim Chloride for Inj IP 1g per vial |
| 101 | Isoxsuprine Hydrochloride Inj USP 5mg |
| 102 | CITICOLINE INJECTION 250 mg/ml |
| 103 | Sterile Piperacillin Sodium and Tazobactam Sodium (81) 2.25g |
| 104 | Sterile Piperacillin Sodium and Tazobactam Sodium (81) 4.5g |
| 105 | Meropenum for injection 500mg & 1g |
| 106 | Xylometazoline HCL Nasal Drops 0.1% & 0.05% |
| 107 | Cetrorelix injection 0.25 mg/vial |
| 108 | Dobutamin HCL Inj 12.55mg |
| 109 | Dobutamin Inj USP 250mg per vial lyophillised |
| 110 | Hyoscine Butyl Bromide Inj BP 20mg |
| 111 | Isoxsuprine HCL Injection USP 5 mg |
| 112 | Clonidine Injection BP 0.15mg /ml |
| 113 | Rabeprazole Sodium for Injection 20 mg |
| 114 | IOPROMIDE INJECTION USP (300 MGI/ML) |
| 115 | CARBOXYMETHYL CELLULOSE SODIUM Eye drops 0.5% |
| 116 | DIATRIZOATE MEGLUMINE AND DIATRIZOATE SODIUM INJECTION USP |
| 117 | Mensa injection 400mg per 4 ml |
| 118 | ACETYL CYSTEINE SOLUTION USP 200 mg |
| 119 | DORIPENEM FOR INJECTION 500 mg |
| 120 | Pentoprazole For Inj 4omg --Pentoking |
| 121 | Oxytocin Inj BP 10IU/ml |
| 122 | Diclofenac Sodium Inj 25mg per ml |
| 123 | GENTAMICIN SULPHATE INJECTION BP 40MG/ML |
| 124 | Iopamidol Injection USP 300mg |
| 125 | Iopamidol Injection USP 370mg |
| 126 | IRINOTECAN HYDROCHLORIDE FOR INJECTION 100MG/5ML |
| 127 | Droperidol Inj USP 2.5mg/ml |
Cream Ointment & Gel 
No. |
Product ( Cream Ointment & Gel ) |
| 1 | Diclofenac Gel BP 1% |
| 2 | DERMAZONE-BG (Gentamicin + Beclomethasone + Clotrimazole Cream) |
Tablet & Capsules 
No. |
Product ( TABLET & CAPSULES) |
| 1 | FLUTAMIDE TABLET USP 250MG |
| 2 | MERCAPTOPURINE TABLETS B.P. 50 mg |
| 3 | CYPROHEPTADINE TABLET BP 4MG |
| 4 | METHOTREXATE TABLETS B.P. 2.5 mg |
| 5 | Misoprostol 200 mcg Tablet |
| 6 | TAMOXIFEN TABLETS BP 20 mg |
| 7 | Amoxicillin Capsule BP 500mg |
| 8 | Ampicillin capsule BP 500mg |
| 9 | Cloxacillin Sodium Capsule USP 500mg |
| 10 | Capecitabine Tablets USP 500 mg |
| 11 | CEPHALEXIN CAPSULE B.P. 500 mg |
| 12 | Metformin Hydrochloride Tab 500 mg Extended release |
| 13 | Artemether 20mg and Lumefantrine 120mg Tablets |
| 14 | Leflunomide tab 20mg IH |
| 15 | Doxasosin Tab USP 2mg |
| 16 | CABERGOLINE TABLETS USP 0.5 mg |
| 17 | HYDROXYUREA CAPSULES 500 MG |
| 18 | Letrozole tablet 2.5mg |
| 19 | Mefloquine Hydrochloride Tablets USP 250 mg |
| 20 | Naltrexone Hydrochloride Tablets USP 50 mg |
| 21 | TAMOXIFEN CITRATE TABLETS B.P.20mg |
| 22 | THALIDOMIDE CAPSULES USP 100 mg |
| 23 | Pregabalin Capsules 75mg |
| 24 | CLOPIDOGREL TABLETS USP 75 mg |
| 25 | Perindropil Tab 4mg |
| 26 | Sulfamethaxazole & Trimethoprim Tab ( Co-Trimazole tab BP ) |
| 27 | Clotrimazole Vaginal Ovules USP 100 mg |
| 28 | Ranitidine Tablets B.P. 150 mg |
| 29 | Ranitidine Tablets B.P. 300 mg |
| 30 | Valsartan Tablets USP 160 mg |
| 31 | Valsartan Tablets USP 320 mg |
| 32 | Valsartan Tablets USP 80 mg |
| 33 | CEFALEXIN CAPSULES BP 250mg |
| 34 | CEFALEXIN CAPSULES BP 500mg |
| 35 | Tibolone Tablets 2.5 mg |
| 36 | Desogestrel 0.15 mg and Ethinylestradiol 0.02 mg USP Tablets |
| 37 | Desogestrel 0.15 mg and Ethinylestradiol 0.03 mg USP Tablets |
| 38 | Levonorgestrel BP 0.75 mg Tablets |
| 39 | Levonorgestrel BP 1.5 mg Tablets |
| 40 | Levonorgestrel 0.10 Mg & Ethinylestradiol 0.02 Mg Tablets BP |
| 41 | Levonorgestrel 0.15 Mg & Ethinylestradiol 0.03 Mg Tablets BP |
| 42 | Levonorgestrel 0.25 Mg & Ethinylestradiol 0.05 Mg Tablets BP |
| 43 | Medroxy Progesterone Acetate Tablets USP 10 mg |
| 44 | MEGESTROL ACETATE 40 MG tablet USP |
| 45 | Pyridostigmine Tablets BP 60 mg |
| 46 | CIPROFLOXACIN TABLET BP 500 mg |
| 47 | CIPROFLOXACIN TABLET BP 250 mg |
| 48 | Carbimazole Tablet BP 5mg |
| 49 | Valsartan 80mg and Hydrothiozide 12.5mg tab USP |
| 50 | Sildenafil tablet 50 mg |
| 51 | Alendronate Sodium tablet 70mg |
| 52 | Pravastatin Tablets BP 40mg |
| 53 | Nitroglycerin tablet 0.3mg |
| 54 | THALIDOMIDE CAPSULES USP 50 mg |
| 55 | Metformin Hydrochloride Tab BP 500 mg |
| 56 | Metformin Hydrochloride Tab BP 850 mg |
| 57 | Azithromycin Tab 250mg USP |
| 58 | Genfitnib Tablets 250mg IH |
| 59 | Erlotinib Tab 100mg & 150mg |
| 60 | FLUCLOXACILLIN CAPSULES BP 500 MG |
| 61 | Frusemide Tablet 40mg |
| 62 | Spiramycin Tablet 1.5MIU |
| 63 | Spiramycin Tablet 3.0MIU |
| 64 | Danazole Capsule 200mg |
| 65 | Paracetamol Tablets 500mg |
| 66 | Amlodipine Besylate Tablets USP 5 mg |
| 67 | Levothyroxin tab BP 25mcg |
| 68 | Levothyroxin tab BP 50mcg |
| 69 | Levothyroxin tab BP 100mcg |
| 70 | Rifampicin Capsule USP 150mg |
| 71 | Tranexamic Acid Capsules 500 mg |
| 72 | LETROZOLE TABLETS USP 2.5 MG |
| 73 | Gefitinib Tab 250mg |
| 74 | Carisoprodol Tablets USP 350 mg |
Liquid Oral 
No. |
Product ( LIQUID ORAL) |
| 1 | Amocicillin Oral Suuspension BP 250mg /5ml |
| 2 | Calcium Polystyrene Sulfonate Powder for Suspension |
| 3 | Sodium Polystyrene Sulfonate Powder for Suspension USP |
| 4 | Povidone Iodine Topical Solution USP 10 % w/v |
| 5 | Allumina , Magnesium & Simeticon Suspension |
| 6 | Isoflurane, Concentrate for Inhalation Solution |
| 7 | Isoflurane, Concentrate for Inhalation Solution |
| 8 | Sevoflurane Solution 99.99% |
Drug Shortage
Registered Office
- 1204 & 1302, Rupa Solitaire ,
Plot No A-1, Sector – 1,
MBP, T.T.C. Indl, Mahape,
Navi Mumbai-400701 - 022 4065 9200 (Board)
- 022 4065 9228 (Marketing)
- flagship@flagshipbiotech.com
Quick Inquiry
